The iTC-200 is a micro-scale (200µl) ITC apparatus designed to make very precise calculations of the enthalpy changes produced upon titration of one analyte into the other (typically to study the binding of small molecules to larger macromolecules).
Isothermal Titration (micro)Calorimetry is a quantitative technique that can determine the dissociation constant (Kd), enthalpy change (ΔH) and stoichiometry (n) of the interaction between two molecules in solution. From these initial measurements, Gibbs free energy changes (ΔG) and entropy changes (ΔS) can be determined.
ΔG = −RTlnKd = ΔH − TΔS
(R is the gas constant and T is the absolute temperature).
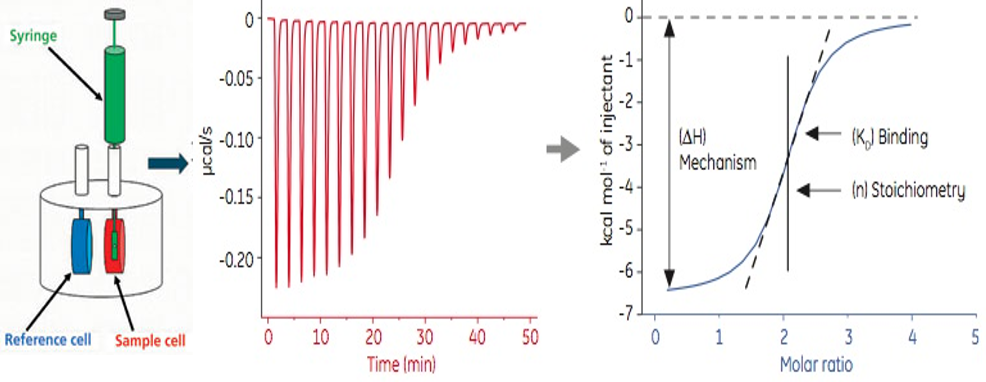
For accurate measurements of binding affinity, the curve of the thermogram must be sigmoidal.
Good experimental design being essentially defined by the c-value… 5 < c < 500
where c = nKaP (P being the protein concentration).